
Macdonald Research Group
>Research<
Teaching Publications Links Home PageI worked on a large variety of projects with Alan H. Cowley's research group at the University of Texas including:
The synthesis and chemistry of main group metallocenium cations:
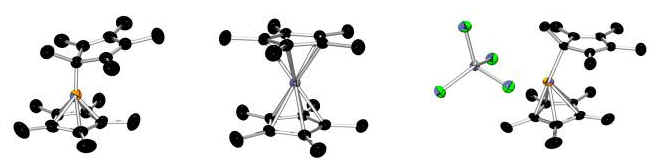
The metallocenium cations of the group 13 elements are very different from sandwich compounds containing transition metals. The isovalent series depicted above (B, Al, and Ga) displays structural differences that are without precedent and the catalytic properties of the cations are highly dependent on the identity of the central metal atom.
The ligand chemistry of +1 oxidation state group 13 compounds (with J. D. Gorden):
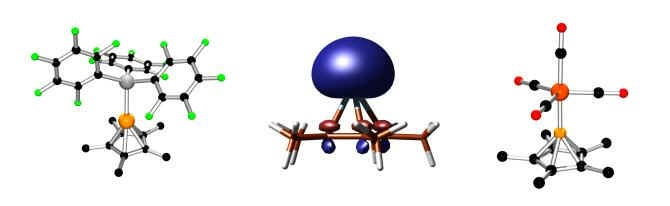
Compounds containing group 13 elements in the normal +3 oxidation state are usually Lewis acids (electron acceptors), however the Cp* ligand allows for the stabilization of +1 oxidation state compounds that are Lewis bases (electron donors). The middle picture depicts the highest occupied molecular orbital (HOMO), which corresponds to the "lone pair" of electrons that is suitable for donation to main group acceptors (left) or transition metal complexes (right).
Understanding the stabilization of persistent radicals (with R. J. Wiacek):
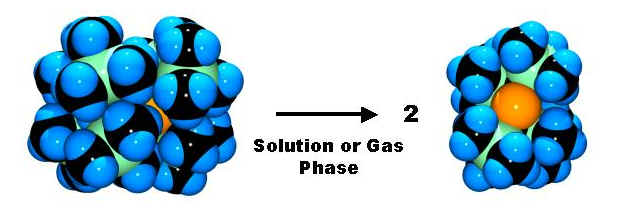
Sterically demanding (bulky) substituents can be used to reverse typical chemical reactivity and stabilize highly-reactive species. Generally, compounds of the form R2Pn-PnR2 are stable and have a strong bond between the two pnictogen atoms. When R is a particularly bulky group with the correct shape (it is CH(SiMe3)2 in the picture above), the molecule cleaves itself into two radicals that are stable indefinitely. The studies we performed (in collaboration with several other groups including those of Rankin, Lappert and Power) provided new insight into how such bulky groups stabilize reactive compounds.
Transition metal carbene and imidazolium cation chemistry (with P. Shukla):
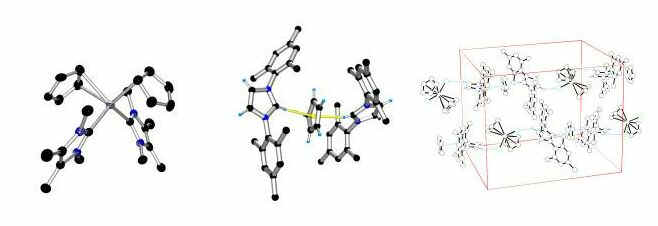
N-heterocyclic carbenes are strong ligands for transition metal complexes. Such donors can have drastic effects on the structure and chemistry of the resultant complexes as demonstrated by the eta2,eta1-manganocene complex (left). During our studies, we discovered a surprising complex containing remarkably strong C-H---p hydrogen bonding (middle and right); the reactivity we discovered has important implications for chemistry carried out in ionic liquid solvents.
The synthesis of main group "constrained geometry" complexes (with J. M. Pietryga):
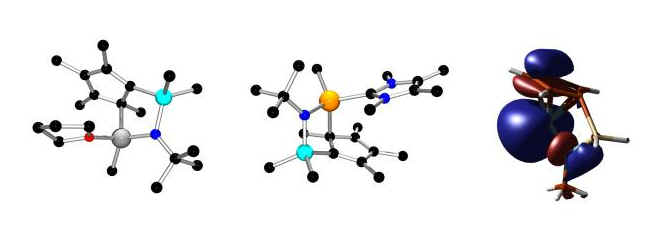
We synthesized the first main group "constrained geometry" complexes. The transition metal analogues are used industrially as catalysts for olefin polymerization. The THF and carbene adducts of aluminum and gallium complexes are shown (left and center) and the calculated lowest unoccupied molecular orbital (LUMO) of a cationic derivative shows that it is a metal-based acceptor (right).
The synthesis of inverse-sandwich and multi-decker main group metallocenes (with J. S. Silverman and J. N. Jones):
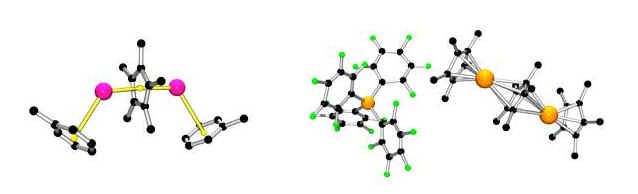
We developed a general method for the synthesis of inverse sandwich and multi-decker main group metallocenes. The inverse sandwich cation [InCp*In] has very weak eta6 interactions with two toluene molecules (left) and the first triple-decker main group cation has interesting behaviour in solution. The "building-block" approach is applicable to many other systems.